Contents
Summary
Shelf-life is a critical factor for bakery manufacturers as it directly impacts product quality, consumer satisfaction, and overall business success. Ensuring an adequate shelf-life allows manufacturers to maintain product freshness, taste, and texture over an extended period, reducing the likelihood of spoilage and food waste. Many manufacturers tackle shelf-life issues by using preservatives such as potassium sorbate or calcium propionate, however the effectiveness of these preservatives is influenced by the end-product pH level. Increasing the shelf-life of a cake can be as easy as changing the baking powder.
What PELL™ Preservit Can Do for You
- Produce a notable increase in active preservative, without impacting volume and shape.
- Extend shelf-life with reduced preservative levels.
- Increase profit margins by reducing raw material costs.
- Give a brighter, more attractive crumb.
Our shelf-life enhancing PELL™ technology is also available for applications such as doughnuts, crumpets, pancakes and many more. To find out more about our range and their capabilities, contact our experts.
Background
It is estimated that the UK food and drink sector produces 1.7 megatonnes of waste per year, with 10% attributed to the bakery sector. With sustainability initiatives and customers being increasingly aware of these numbers, bakery manufacturers are looking for ways to reduce their waste. By prioritising shelf-life in bakery products, manufacturers can not only meet sustainability goals but also uphold product integrity, increase profitability, and ultimately, sustain long-term competitiveness in the market.
Challenge
Generally, due to the ingredients used, a cake will sit around the pH of 7.0. In manufactured cakes, preservatives like potassium sorbate and calcium propionate are used because they are effective within the pH range of 4.6-7.0. As the cake is baked, these preservatives dissolve in the cake batters to form weak acids. These can only inhibit microbial growth if they remain in an undissociated, or ‘active’, state.
The more acidic the cake environment, the more ‘active’ the preservative becomes. However, manipulating the pH in baked goods can be challenging. It would typically require either the addition of excess leavening acid or a complete recipe reformulation; both of which can seriously impact taste, texture and volume.
Solution
Using PELL™ Preservit enables a significant reduction in the pH of baked goods and therefore optimises the performance of the preservatives used. This means no recipe reformulation is required to maintain your signature taste, texture and volume.
Appearance
Making the change to PELL™ Preservit resulted in a final product that showed additional benefits to the pH reduction, by producing a cake with a consistently brighter, more attractive crumb.
Standard baking powder

pH 7.0
PELL™ Preservit baking powder

pH 6.3
Fig. 1. Loaf cakes soaked with Universal Indicator show the pH reduction achieved with PELL™ Preservit baking powder. Values confirmed using laboratory pH meter.
Fig. 2. pH scale showing where the product pH lies.
PELL™ Preservit achieved a pH reduction of 0.7 in the final cake, which equates to an estimated 400% increase in active preservative.
Increased Effectiveness of Potassium Sorbate as pH Decreases From 7
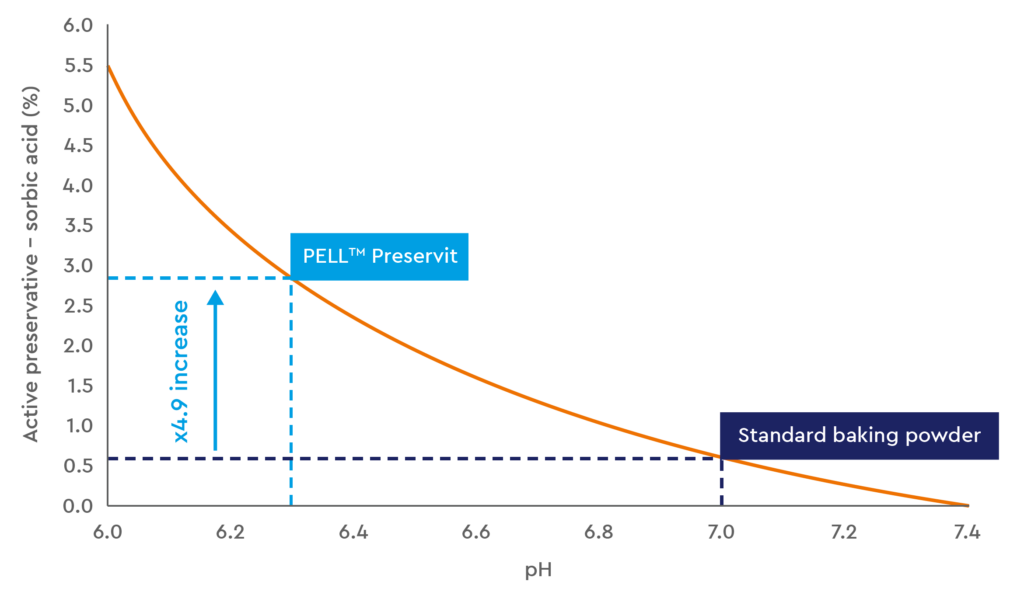
Fig.3. Effect of reducing the end product pH on the active potassium sorbate, as derived from the Henderson-Hasselbalch equation.
Having the ability to reduce the pH of the product will drastically increase the active preservative level, as demonstrated above in Figure 3, improving shelf-life at no additional cost. Alternatively, customers have the option to lessen the level of preservative inclusion to not only save on raw material costs, but produce a cleaner overall end-product. The table in Figure 4 explains the reduction in preservatives that is achievable the through pH reduction.
Reduction in Preservative Achievable as pH Decreases From 7
| pH | Reduction in potassium sorbate required (%) |
| 7.0 | ~0 |
| 6.8 | 36.7 |
| 6.6 | 59.8 |
| 6.4 | 74.5 |
| 6.2 | 83.7 |
| 6.0 | 89.5 |
Fig. 4. Decreases in the required quantity of sorbic acid in relation to the end product pH reduction, as derived from the Henderson-Hasselbalch equation.
Thanks to rigorous on-site raw material and batch tests in our laboratory, you can rest assured that our products will perform well every time.









